Abstract
Introduction: Leukemic stem cells (LSCs) are responsible for relapse in many patients with acute myeloid leukemia (AML). CD34+CD38- stem cells (SC) have been described as the most therapy-resistant subpopulation, although different cell compartments may contain LSCs. LSCs express different markers, CD123 being one of the most often aberrantly expressed, while the expression of CD90 remains controversial.
The aim of our study was to analyze the prognostic value of the SC presence at diagnosis in AML patients and to find out which markers could better identify LSC and their influence in prognosis.
Methods: 136 patients with AML diagnosed between may'13 and june'21 and candidates to intensive treatment were included in the study.
Bone marrow samples were analyzed identifying SC as CD34+CD38- by second generation flow cytometry using Infinicyt software, v. 2.05. We used Euroflow recommendations for AML studies and a specific tube with the following markers: CD34, CD38, CD45, CD123, CD90, CLL-1, CD117 y HLADR. Statistical analyses were performed using SPSS v. 26. We considered as "event" the absence of response after induction, relapse or death.
Results: The patient cohort consisted of 64 males and 72 female AML patients, with a median age of 54 years (0-76). Overall survival (OS) at 2 and 5 years were 43% and 35%. CD34+CD38- SC were identified in 114 (84%) cases. Risk subgroups according to the European Leukemia Net (ELN) were as follows: 39 favorable [30 (76.9%) with SC], 46 intermediate [37 (80.4%) with SC] and 51 high risk [47 (82.5%) with SC] (p>0.05).
Eleven out of 18 (61%) patients without SC reached complete remission (CR) with negative measurable residual disease (MRD) vs 40 out of 86 (46.5%) patients with detectable SC (p=0.2).
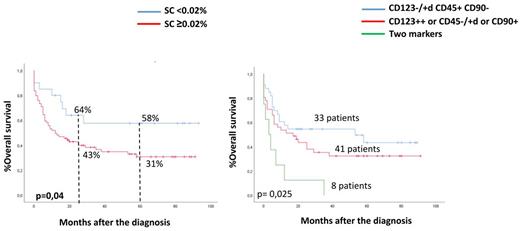
Leukemic cells were CD34 + in 100 patients and CD34- in 36. Among those patients with CD34+ blasts, event free survival (EFS) at 2 and 5 years were 56% and 56% for those with < 0.02% SC (1st quartile) vs 34% and 25% in the > 0.02% SC group (p= 0.1). OS at 2 and 5 years were 64 and 58% as compared to 43% and 31% for patients with < 0.02% or > 0.02% SC, respectively; p=0.04). No significant differences were observed among patients without CD34+ blasts (Figure 1).
Considering only patients in CR, EFS at 2 and 5 years for the group with < 0.02% SC was 83% and 83% as compared to 59% and 43% for those with > 0.02% SC (p=0.031), while OS at 2 and 5 years was 85% and 85% versus 62% and 49% for patients below or above that threshold, respectively (p= 0,065).
Next, we evaluated the expression of LSC markers (CD123, CD90 and/or CD45) on SC, and we identified 3 different groups of patients: group A (no LCS expression, n=33), group B (expression of one of these LSC markers, n=41) and group C (expression of 2 or more. n=8). The EFS at 2 years was 52%, 29% and 13%, respectively (p=0.02); OS at 2 years was 55%, 43.5% and 12.5%, respectively (p=0.025) (Figure 2).
Conclusion: Based on these data, the presence of SC > 0.02% has a prognostic impact in AML patients with CD34+ blasts. With the combination of 3 markers, LSC can be identified within SC with prognostic value.
Disclosures
Perez-Simon:GILEAD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ABBVIE: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; PFIZER: Research Funding; JAZZ: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ALEXION: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses.
Author notes
Asterisk with author names denotes non-ASH members.